Reverse Osmosis can be better understood by first understanding osmosis.
Osmosis means a subtle or gradual absorption.
When water flows from a less concentrated solution through a semi-permeable membrane to a more concentrated saline solution until concentrations on both sides of the membrane are equal, that is known as natural osmosis.
Osmosis is a special case of diffusion in which the molecules are water and the concentration gradient occurs across a semi-permeable membrane.
Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration.
In other words, we may say that it is the spreading of molecules from a higher level to a lower level.
The semipermeable membrane allows the passage of water, but not ions or larger molecules (e.g., glucose, urea, bacteria).
Diffusion and osmosis are thermodynamically favorable and will continue until equilibrium is reached.
When sufficient pressure is applied to the membrane from the ‘concentrated’ side of the membrane, Osmosis can be slowed, stopped, or even reversed.
So, How does reverse osmosis work?
Imagine a semi-permeable membrane with fresh water on one side and a concentrated aqueous solution on the other side.
If normal osmosis takes place, the freshwater will cross the membrane to dilute the concentrated solution.
In reverse osmosis, the pressure is exerted on the side with the concentrated solution to force the water molecules through the membrane to the freshwater side.
In reverse osmosis, the idea is to use the membrane to act like an extremely fine filter to create drinkable water from salty or contaminated water.
The seawater is put on one side of the membrane and pressure is applied, to stop and then reverse the osmotic process.
It generally takes a lot of pressure and is fairly slow, but it works.
Reverse osmosis is a percent rejection technology.
The purity of the product water depends on the purity of the inlet water.
The purity of reverse osmosis product water is much higher than the purity of the feed water.
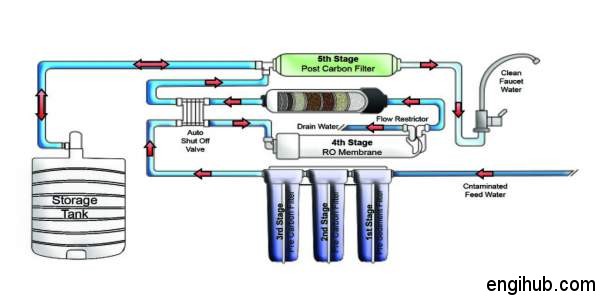
How does Residential Reverse Osmosis System Works?
Clean water passes through and impurities that are too big to pass through the membrane are left behind and flushed away.
01) Tap water is passed through a particle filter that removes silt and large particles.
02) Next, an activated carbon filter traps minerals and contaminants.
03) Water enters the Reverse Osmosis module under pressure, allowing only clean water to pass through.
04) Treated water is passed through an activated carbon filter and is ready for use.
To desalinate seawater, Reverse Osmosis is one of the best options available in water engineering. Sometimes reverse osmosis is used to purify liquids in which water is an undesirable impurity.
Reverse osmosis is often used in commercial and residential water filtration also.
Reverse osmosis can also remove dissolved minerals that cause hardness, toxic chemicals, and other impurities.
It can improve the taste of your water. This unit may also remove contaminants such as chromium, mercury, fluoride, and nitrates.
Besides this information, you are suggested to read something more from below engineering books
So, for a detailed study on this topic, I recommend reading these books, Recycle of Papermill Waste Waters and Application, R O Water Purification Setup and Operation
If you like the post, share it with your friends and also on social sites.



